Abstract
Introduction: Although the therapeutic landscape for multiple myeloma (MM) has expanded, MM is an incurable disease and treatment of patients (pts) with relapsed or refractory multiple myeloma (RRMM) remains challenging. Pts triple refractory to immunomodulators (IMIDs), proteasome inhibitors (PIs), and anti-CD38 antibodies have few therapeutic alternatives and poor prognosis. Belantamab mafodotin (blmf) is a first-in-class antibody-drug conjugate targeting B-cell maturation antigen (BCMA) and showed promising results in the DREAMM-2 trial. The aim of the ALFA study is to describe blmf effectiveness and safety in pts with RRMM in a real-life setting.
Methods: ALFA is a non-interventional, retrospective study of pts with RRMM initiating blmf in 46 centers in France during early access programs from April 27, 2020, to June 30, 2021. Pts characteristics, overall response rate (ORR, ≥partial response [PR]), clinical benefit rate (CBR, ≥minimal response [MR]), ≥very good PR (VGPR) rate, progression-free survival (PFS), overall survival (OS), and safety were assessed. Subgroup analyses for PFS and OS were performed according to best response, CBR, cytogenetic risk, renal failure, age at blmf initiation, previous penta-exposition (at least 2 PIs, 2 IMIDs, and 1 anti-CD38 exposition), and number of prior lines of treatment.
Results: Between April 2020 and June 2021 (median duration of follow-up 7.8 months [mo]), 184 pts initiated blmf. Median time from MM diagnosis to initiation of blmf was 6 years, and 107 pts (58%) had received ≥5 prior lines of therapy. At initiation, 53% were male, median age was 70 years with 30% of pts aged of 75 years or older; 49% had renal failure; 79% were penta-exposed; and 8% had extramedullary disease. Among pts with available data (n=156), 36.5% had an ECOG PS ≥2. Cytogenetic profiles at initial diagnosis were available for 87 pts (47%), among whom 27 (33%) had high cytogenetic risk defined as pts presenting either t(4;14) or del(17p) or t(14;16); 40% had ophthalmological history (cataract in 53%). Blmf was delivered by intravenous infusion in 21-days cycles according to SmPC. Median dose at initiation was 2.5 mg/kg (min max 1.6-3.0). The median number of blmf cycles received was 3 (Q1-Q3 2-7).
The ORR was 35% (≥VGPR 21%, PR 14%) and 59 pts (39%) achieved clinical benefit; 25% had stable disease (SD) and 37% had progressive disease (PD).
The median PFS (mPFS) for the whole population was 2.7 mo (95 CI% 1.9-3.3), and median OS (mOS) was 9.5 mo (95 CI% 7.2-11.9). For both OS and PFS, no differences were found in subgroups according to cytogenetic risk, renal failure, age at blmf initiation, penta-exposition, and number of prior lines.
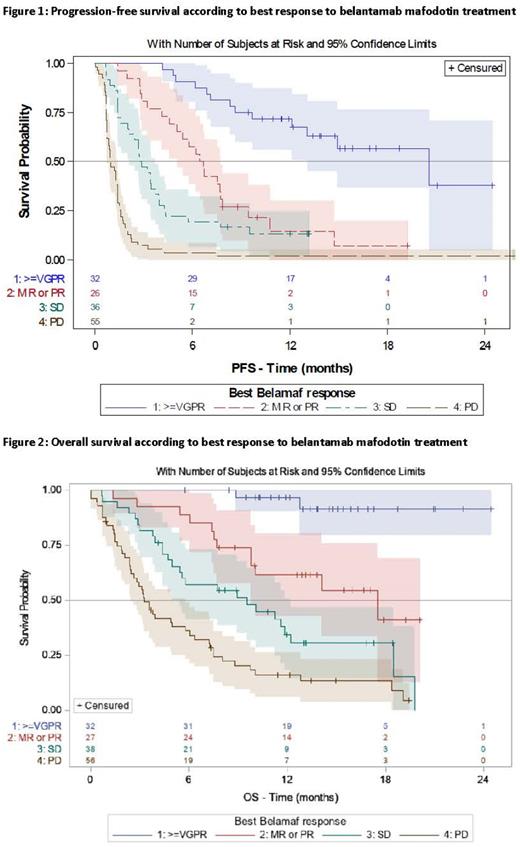
According to best response, mPFS was 20.6 mo (95 CI% 13.1 to not reached) in pts with ≥VGPR, 6.6 mo (95 CI% 5.1-7.7) in pts with PR or MR and 2.8 mo (95 CI% 2.1-3.7) in pts with SD; P<0.01 (Figure 1). mOS was not reached in pts with ≥VGPR (1-year survival rate 96.7% [95 CI% 90.2-100.0]), 17.5 mo (95 CI% 9.8 to not reached) in pts with PR or MR, 9.5 mo (95 CI% 5.4-12.2) in pts with SD, and 3.3 mo (95 CI% 2.5-5.8) in pts with PD; P<0.01 (Figure 2). In pts with a clinical benefit, mPFS was 9.7 mo (95 CI% 7.5-14.9) and mOS was not reached.
Safety findings were consistent with the known profile of blmf. Adverse events (AEs) were reported in 159 pts (86.4%). Most frequent reported AEs were ocular AEs in 56.0% of pts (n=103), among whom 70 pts had keratitis/keratopathy, and thrombocytopenia in 13.0% of pts. Infusion reaction was reported in 2.7% of pts. Permanent discontinuation due to AEs occurred in 12.5% of pts. Ocular AEs led to dose modification, temporary interruption, and permanent discontinuation in 19.6%, 11.4%, and 12.0% of pts, respectively.
Conclusions: The ALFA study confirms the results of belantamab mafodotin as reported in the DREAMM-2 trial. In the overall population, the ORR was 35% (21% with ≥VGPR), CBR was 39%, mPFS was 2.7 mo, and mOS was 9.5 mo. In patients with a clinical benefit, mPFS was 9.7 mo and mOS was not reached. No new safety concerns were identified. These data presented from the largest real-life study conducted to our knowledge confirm previous results.
Funding: GSK (214820)
Disclosures
Roussel:GSK, Takeda, Janssen: Honoraria. Texier:Kappa Sante: Current Employment. Germain:Kappa Sante: Current Employment. Sapra:GSK: Current Employment, Current equity holder in publicly-traded company. Paka:GSK: Current Employment, Current equity holder in publicly-traded company. Kerbouche:GSK: Current Employment, Current equity holder in publicly-traded company. Colin:GSK: Current Employment, Current equity holder in publicly-traded company. Leleu:Amgen: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; Pfizer: Honoraria; BMS: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal